From the original article on September 27, 2021. Author: eugyppius.
A pre-print by researchers at Osaka University in Japan studies the specifics of Delta immune-escape adaptations, and brings more ominous news: The Delta strains now circulating have obvious vaccine-escape adaptations. In some ways, these adaptations have enhanced the potential infectiousness of Delta. But this potential has not yet been realised, because the Delta spike protein has not fully escaped. When that escape does happen, antibodies against legacy spike elicited by the BioNTech/Pfizer vaccines will probably enhance Delta’s transmissibility.
To understand how this can be true, we must take a closer look at spike:
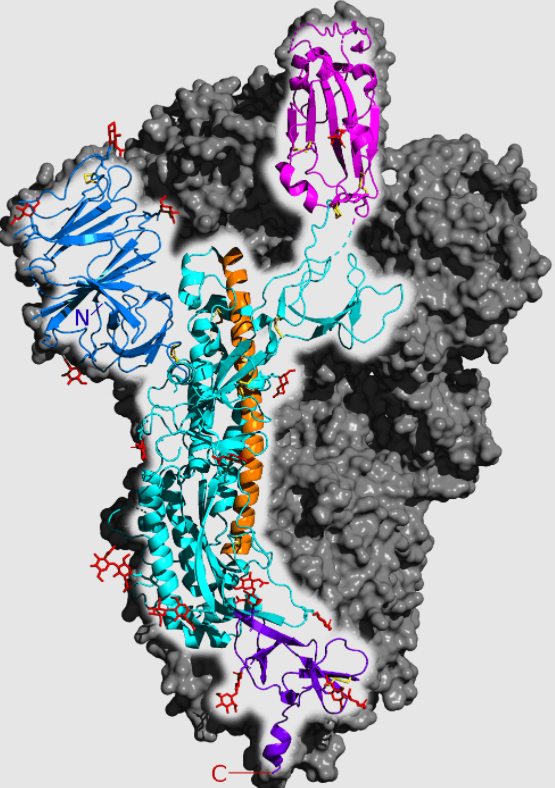
The pink/magenta region at the top is the ACE2 receptor binding domain, or RBD. The RBD interacts with the ACE2 protein expressed by cells in our lungs. Think of it as a key that SARS-2 uses to enter your cells. The vaccines elicit anti-RBD antibodies that interfere with ACE2 interactions. It’s the equivalent of changing your locks, and now Delta has been forced to try a different key: It has two distinctive RBD mutations, but these mutations are not unique to Delta. We also find them in other variants, which are less successful than Delta, so it looks like they’re not crucial to Delta’s success.
The preprint researchers note, however, that Delta appears to have special, unique enhancements to the N-terminal domain (NTD), which is the blue area on the upper left of this spike diagram. “This suggests,” they say, “that mutations in the NTD may play a key role in the high infectivity of the Delta variant.”
Our vaccines elicit antibodies against both of these domains, but so far most attention has been directed against anti-RBD antibodies. Researchers, however, have shown that antibodies against the (blue) NTD play an equally crucial role, and that the wrong anti-NTD antibodies change the configuration of the RBD and actually enhance its capacity to bind to ACE2. Here a simple schematic from another article by the Osaka researchers:
Basically, SARS-2 virions have receptor binding domains that are not fully optimised always and everywhere for binding to ACE2. The right kinds of antibodies, however, can force the NTD and then the RBD into a more ideal configuration and enhance infectiousness, as you see on the right side of this image.
Your immune system, in other words, deploys a variety of antibodies against the NTD. Some of them neutralise it. These are the ones you want. Others, however, merely force NTD and thereby RBD into a more infectious configuration. These are the ones you don’t want. The enhancing antibodies aren’t a problem if you also have neutralising antibodies against NTD and RBD to counteract any advantage.
Back to the preprint. The authors ask how our vaccine antibodies against legacy spike hold up to the new and improved Delta NTD:
We analyzed … 16 anti-NTD neutralizing antibodies, and found that none of the anti-NTD neutralizing antibodies could recognize Delta spike … In contrast, when we analyzed the binding of the anti-NTD infectivity-enhancing antibodoies … eight out of ten anti-NTD enhancing antibodies bound to Delta spike at levels comparable with wild-type spike …
The situation, then, is precisely this: The vaccines gave billions of people antibodies to legacy spike proteins, including anti-NTD neutralising antibodies and anti-NTD enhancing antibodies. Delta is evolving to escape the neutralising antibodies, but not the enhancing antibodies. Which is what you would expect. We have sown antibodies against one viral protein in the lungs of half of mankind, and that protein has now evolved slight modifications to use those antibodies to its advantage where it can, and to escape the rest.
Remember, we are still talking about a single domain in the complex spike protein: There are other antibodies to RBD that still disadvantage Delta. The net effect is still that all of these antibodies, added together – particularly those against the RBD – hurt rather than help.
There are, however, Delta strains with key escape RBD mutations. The authors of this preprint find that four such mutations, already out there in various Delta sub-strains, are sufficient for Delta to evade the anti-RBD antibodies more or less completely.
In sum: Beneath the surface, Delta has evolved to use anti-NTD antibodies, like those elicited by BioNTech/Pfizer vaccines, to enhance its infectiousness. So far we have not seen any effect, because the BioNTech/Pfizer vaccines still elicit anti-RBD antibodies that hurt Delta on balance. But, all the mutations necessary to escape these crucial anti-RBD antibodies exist. All that remains is for a single Delta strain to pick up the right combination of them, and BioNTech/Pfizer antibodies will provide “weak neutralization only at the highest concentration”. Otherwise, they will enhance infectiousness.
UPDATE: Some objections about the in vitro approach of the study authors by Brian Mowrey (who writes Unglossed) in comments here. While I don’t share all of his reservations, he’s right that the significance of these findings for the broader immune response of the vaccinated to potential future Delta escape strains is unclear.
Library of Chadnet | wiki.chadnet.org